Mesenchymal Stem Cells: The Future Of Rehabilitation
What Are Mesenchymal Stem Cells?
Mesenchymal Stem Cells are a unique type of connective tissue that is only partially developed and can further specialize into specific forms of connective tissue. Multipotent is a scientific term for cells that can further evolve into different cell types.
You will often find this particular form of Multipotent cell referred to as an MSC. MSCs are also referred to as Marrow Stromal Cells when scientifically 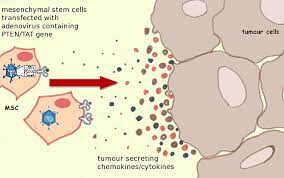 appropriate.
appropriate.
These cells can develop into a number of different types of cells, including fat cells, cartilage cells, and bone cells. As you can imagine, there is a growing interest in cultivating Mesenchymal Stem Cells for use in many different medical treatments, especially those related to bone and joint injuries. The ability for MSCs to develop into more complex tissues has been scientifically proven both in a laboratory environment and in animal subjects.
Why Are They Called Mesenchymal Stem Cells?
As researchers learn more about these cells, coming up with an optimally appropriate name became increasingly important:
Mesenchyme is a preliminary form of connective tissue which evolves in the embryo stage of development, but this name does not fully meet the needs of MSCs. This tissue forms in the central layer of the embryo, known as the mesoderm. Further in development, Mesenchyme evolves into both connective and hematopoietic tissue. In contrast, Mesenchymal Stem Cells can only develop into connective tissue, which proves that Mesenchyme and MSCs are related cells, but different.
Stromal Cell refers to a different aspect of MSC function. These cells are a form of connective tissue that provides structural support to a larger cell. Stromal Cell is accurate enough a term to describe one way that the body uses MSCs, but it does not encapsulate the complete form and function of the cell. This is because it has only recently been determined that MSCs can facilitate the healing of damaged tissue, necessitating a broader set of naming conventions for the cellular type. This is why cells that were once almost universally described as Marrow Stromal Cells are now commonly referred to as MSCs by researchers. Now it is clear that these MSCs exist throughout the body in various tissue forms, including corneal stroma, adult muscle, adipose tissue, and umbilical cord blood. They even exist in the core of the baby teeth. Despite their multipotent configuration, they cannot recreate a whole organ, which is why the new naming convention Multipotent Stromal Cell is becoming more common.
When do Mesenchymal Stem Cells Develop?
The earliest form of MSCs manifest during prenatal development can be found in the tissue of the umbilical cord, specifically in the cord's blood and in Wharton's Jelly.
Although MSCs are present in the umbilical cord's blood, they are in greater concentrations in Wharton's Jelly, which is also a significant source of stem cells that develop into blood cells of various types, known as Hematopoietic Stem Cells.
MSCs Found in the Umbilical Cord
Today, umbilical cords are simply discarded after a successful pregnancy. Still, there is growing evidence that these umbilical cords may be a fantastic and safe method of obtaining these primitive MSCs for medical research and treatment.
Mesenchymal Stem Cells Found in the Molars
Another area dense in MSCs is the third childhood molar of the lower jawbone. These cells are considered multipotent today, but initial research provides evidence that these cells may actually be pluripotent.
Mesenchymal Stem Cells develop into various tissues, including nervous tissues, dental pulp, blood vessels, dentin, and enamel. All told, there are at 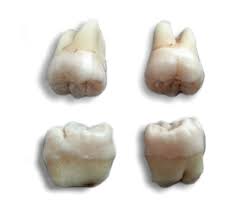 least twenty-nine different organs produced from MSCs.
least twenty-nine different organs produced from MSCs.
Also, because these teeth can generally be harvested before ten years of age, naturally, with negligible mortality risk, there is a high probability that this source of MSCs will become a significant source of MSCs for treatment, research, and that patients may even be recommended to save them for future potential therapeutic use. MSCs also can develop into liver cells.
Mesenchymal Stem Cells Found in the Amniotic Fluid
Another concentrated source of MSCs is in the amniotic fluid of the placenta. Evidence suggests that at least one percent of the cells in the amniotic fluid are pluripotent MSCs.
Mesenchymal Stem Cells Found in Adipose Fat
After childhood, one of the most concentrated sources of Mesenchymal Stem Cells is Adipose Fat Tissue. Medical research has shown that in a single gram of adipose fat has more than five hundred times the MSCs as an equivalent amount of aspirated bone marrow. For this reason, a lot of research is going on right now concerning using MSCs found in fat cells to treat various medical conditions.
Mesenchymal Stem Cells May be Found Within Peripheral Blood Cells
There is some evidence that Mesenchymal Stem Cells may be found in sufficient concentrations in Peripheral Blood Cells, but this evidence is far from conclusive. However, a few studies have been able to extract MSCs from these blood cells and foster them in culture.
The History of Mesenchymal Blood Cells
A medical researcher named Alexander Maximow could single out a particular form of precursor tissue located in the Mesenchyme, which could evolve into a variety of hematopoietic tissue.
Later in the 1960s, two researchers, James Till and Ernest McCulloch, discovered that marrow cells could clone themselves essentially. In the 1970s, a research team led by A.J. Friedenstein could isolate and definitively prove the ability of Stromal Marrow Cells to develop multipotent.
Further study of these cells uncovered that marrow cells had a high level of plasticity and that particular environmental triggers could alter their development into various tissue forms.
For example, if these cells were allowed to develop in the same dish as dexamethasone, inorganic phosphate, and Vitamin C, the marrow would develop into osteoblasts. On the other hand, exposure to TGF-b would induce the cells to develop into chondrocytes.
Morphology of Mesenchymal Stem Cells
MSCs can be physically described as a central body with a few thin and long processes extending out. The body of the call holds a nucleus that is round, large, and has a conspicuous nucleolus. The nucleus is covered in fine particles known as chromatin, which makes it easy to see.
The rest of the cell body is occupied by polyribosomes, mitochondria, rough endoplasmic reticulum, and limited Golgi apparatus. Each of the cells is skinny and long, is dispersed widely, and a small number of reticular fibrils connect the cells.
How Are Mesenchymal Stem Cells Detected
The International Society for Cellular Therapy provides the most established definition for what qualifies as an MSC. They consider MSCs any cell with malleable properties under standard conditions and is structured like a fibroblast.
Some medical researchers even believe that fibroblasts and MSCs are operatively identical.
Also, MSCs can undergo chondrogenic, adipogenic, and osteogenic differentiation in culture. Finally, cultured Mesenchymal Cells also have a particular set of active and inactive enzymes on their surface.
What is the Capacity for MSCs to Differentiate?
Mesenchymal Stem Cells have a tremendous ability to reproduce themselves while not losing their ability to evolve, but there is no easy way to quantify that capacity. The general method to prove multipotency is for cells to evolve into neurons, myocytes, chondrocytes, adipocytes, and osteoblasts with sufficient environmental cues.
Mesenchymal Stem Cells can develop into all these different types of cells, but it is unclear whether creating a functioning neuron from an MSC is possible.
The ability for an MSC to develop into a particular form depends upon the individual cell and how the change is produced, whether via mechanical or chemical means, for example. It is also unclear whether differentiation occurs because of an overall capacity to differentiate or the proclivity of specific cells to differentiate in their own capacity.
There is also evidence that the older a donor is when MSCs are contributed, the slower the cells differentiate and proliferate. There is no definitive evidence regarding whether this is because there are issues in donated MSCs or a lower concentration of MSCs in culture.
Effects of Mesenchymal Stem Cells on the Immune System
Research has shown that in human beings, MSCs block the function of T-Cells and dendritic cells. They also release cytokines, which suppress the immune responses in their immediate environment. These cells alter immune function more vigorously in areas where inflammation is prominent.
In other situations, however, studies have shown that these effects are not universal and can be altered by other extenuating circumstances. Because each isolated culture of MSCs has its unique configuration, the cell cultures often react differently to the same stimulus.
The Culturing of Mesenchymal Stem Cells
There are a number of methods to develop MSC cultures. Still, the most prominent is taking ficoll-purified bone marrow or unadulterated bone marrow and placing the mononuclear cells straight into flasks or culture plates.
After one to two days, MSCs will stick to the specialized plastic, but hematopoietic and red blood cells will not. There is evidence that this technique is not perfect, and at least some MSCs do not adhere to the given time frame.
How Are Mesenchymal Stem Cells Related to Cancer?
MSC proliferation is involved in many forms of cancer, especially those which impact the bone marrow. Hematological Cancers most commonly impact the function of MSCs.
What Are the Medical Uses for Mesenchymal Stem Cells
There are currently methods by which MSCs can be mobilized and activated if necessary. Unfortunately, using today's medical methods, there is limited efficiency. For example, muscles recover quite slowly from injury because of this limited mobilization.
There is promising research that suggests that there may be scientific methods to speed up this healing process.
In recent history, there have been a number of medical success stories involving the transplantation of MSCs directly into the bloodstream in medical conditions such as sepsis. Still, in many of these cases, alternative forms of treatment are more effective, especially in the case of conditions related to  peripheral tissues.
peripheral tissues.
The most effective form of MSC administration appears to be to distribute the cells directly to the area which requires rehabilitation. When delivered directly to the bloodstream, the lungs absorb many of the MSCs, limiting the capacity of the treatment.
MSCs have been used frequently in an orthopedic environment, but these treatments need more rigorous and widespread use before their effectiveness can be fully determined.
One physician, Dr. Wakitani, has released a set of five case studies in which he treated five defective knees by implanting MSCs and quantified the effectiveness of the treatment.
Effectiveness of Cryogenically Preserved MSCs
Researchers have shown that MSCs are highly receptive to cryogenic freezing but slowly regain their efficacy after they've been thawed, meaning they should be allowed to thaw and propagate before implantation for maximum effectiveness.
Many clinical studies have failed explicitly because MSCs were implanted directly after thawed. By allowing the cells to propagate after thawing, they will fully recover from being frozen, with the cells being just as valuable as if they were never frozen.
- Stem Cells [Last Updated On: September 5th, 2024] [Originally Added On: September 22nd, 2020]
- The Potential Role Of Gh-rh In Treating Alzheimer's [Last Updated On: August 29th, 2024] [Originally Added On: September 24th, 2020]
Word Count: 1825







